VIDAS® Lyme panel
Differential diagnosis of Lyme Borreliosis
The combined use of the automated VIDAS® Lyme IgM and VIDAS® Lyme IgG assays will help you provide patients with reliable, differential diagnosis of Lyme borreliosis in just 27 minutes.
- Clear-cut results
- High specificity and sensitivity
- Specific detection of IgG antibodies to Borrelia burgdorferi sensu lato (sl) and in cerebro-spinal fluid (CSF) samples, to aid in the diagnosis of Neuroborreliosis*
Precisa de mais informação?
Transmitted through the bite of infected ticks, Lyme Borreliosis is the most common and rapidly spreading tick-borne disease in the world. However many of the symptoms can also occur with other diseases. VIDAS® Lyme IgM and VIDAS® Lyme IgG allow accurate, rapid, differential diagnosis to avoid misdiagnosis and help prevent progression of the disease towards severe and disabling neurological, cutaneous or articular manifestations.
Clear-cut serological profile
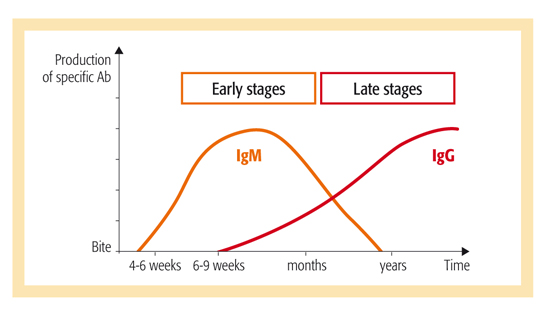
- Separate IgG and IgM results to facilitate classification of Lyme Borreliosis as early or late/chronic, active or immune
- Helps ensure patients receive rapid, optimized treatment depending on their stage of infection
- Easy result interpretation:
- No equivocal zone for VIDAS Lyme IgG results
- Avoids unnecessary patient anxiety and limits additional testing
Detection of immunological response following a bite from a tick
infected with Borrelia burgdorferi
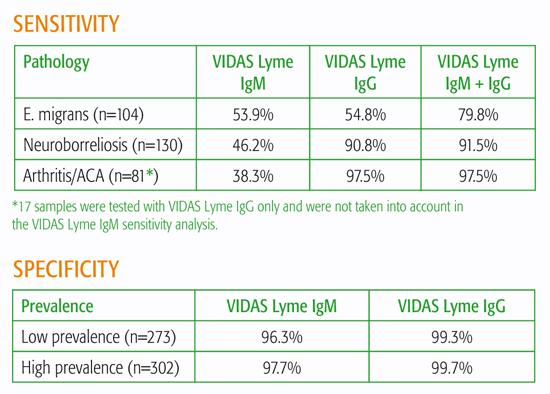
High specificity and sensitivity*
- The tests use innovative recombinant chimeric proteins* (VlsE, DbpA, OspC), allowing detection of all main pathogenic Borrelia strains
- Less rework and confirmatory tests
- Low level of cross-reaction with syphilis and other infectious diseases*
- Only patients with Lyme Borreliosis are treated
* See package inserts for more details
** Performance on serum samples, for references outside US and Canada
*** 17 samples were tested with VIDAS Lyme IgG only and were not taken into account in the VIDAS Lyme IgM sensitivity analysis
Cost-effectiveness and streamlined workflow
VIDAS® Lyme IgM and VIDAS® Lyme IgG are performed on the VIDAS® automated immunoanalyzers, appreciated worldwide for their simplicity, flexibility and accuracy.
Key benefits:
- Ease-of-use
- Rapid results: only 27 minutes
- Protocol compatibility between both tests
- Single-dose format
- Calibration only once every 28 days
| VIDAS® Lyme IgM | VIDAS® Lyme IgG | |
|---|---|---|
| Reference | 30 319* | 30 320* |
| Tests / kit | 60 | 60 |
| Time to result | 27 minutes | 27 minutes |
| Sample type | Plasma, Serum | Plasma, Serum, CSF* |
| Sample volume | 100 µl | 100 µl |
| Calibration frequency | Every 28 days | Every 28 days |
* For references outside US and Canada only
Find more technical details on www.myvidas.com.
Consult your local bioMérieux representative for product availability in your country.
Related Publications
- Usefulness of quantitative IgG assays Borrelia VLSE LIAISON® and the new VIDAS® Lyme for serological follow-up of patients with Lyme borreliosis
O. Péter, R. Lienhard, R. Vonlanthen, A. Bonnet-Pierroz, C. Béguelin, A. Schaller, D. Genne (Switzerland)
Poster | ECCMID 2014
- Performance evaluation of the new VIDAS Lyme IgM and IgG assays compared to the previous VIDAS Lyme total antibodies assay on fresh prospective sera
S. Neifer, O. Wankmüller (Germany)
Poster | ECCMID 2012
- Evaluation of two automated tests on the VIDAS® system to detect anti-Lyme disease antibodies IgG and IgM in human serum, plasma and CSF
G. Bouchard, O. Péter, A. Bonnet-Pierroz, R. Vonlanthen, P. Cosin, N. Ammour, A. M. Brevet, P. Broquedis, N. Dehainault, C. Perret, B. Inçaurgarat (France, Switzerland)
Poster | ECCMID 2010
Useful Links
- European Concerted Action on Lyme Borreliosis (EUCALB)
- Centers for Disease Control and Prevention | Resources for Clinicians
Find more scientific and educational resources on www.myvidas.com.